Aspirin is one of the most famous medications in the world. Its international significance is notable due to the inclusion in the Model list of essential medicines (2022, para. 14) by World Health Organization. Pahan and Pahan (2019, p. 595) refer to Aspirin as “one of the most effective, safe, and economical drugs needed for basic health care”. Although medications with similar effects have existed for centuries, Aspirin has only become popular in the later half of the twentieth century. Despite its near universal acceptance, there are also negative effects, which cause the search for alternative medications.
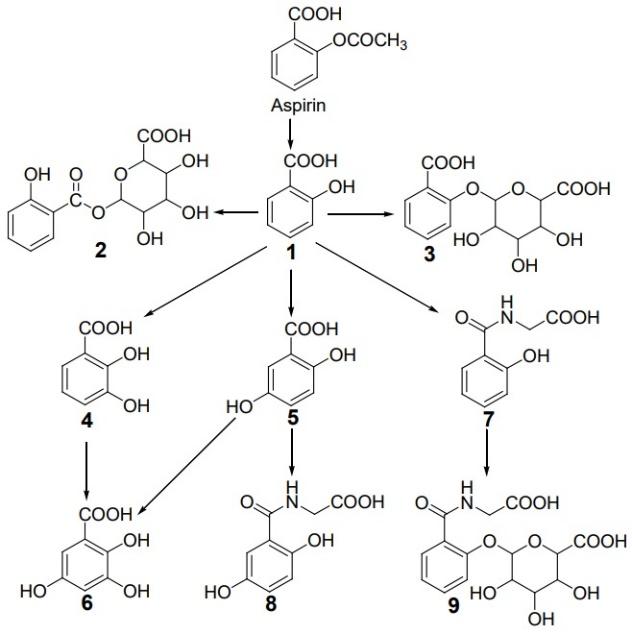
The proper scientific name of aspirin is acetylsalicylic acid. Figure 1 shows chemical structure of Aspirin and the products of its metabolism. Nine major metabolites are depicted:
- salicylic acid,
- salicylacyl glucuronide,
- salicylphenol glucuronide,
- 2,3-dihydroxybenzoic acid,
- gentisic acid,
- 2,3,5-trihydroxybenzoic acid,
- salicyluric acid,
- gentisuric acid, and
- salicyluric phenolic glucuronide (Uzzaman, Chowdhury and Hossen, 2019, p. 1).
When Aspirin interacts with molecules that cause inflammation, it chemically binds the enzymes and stops the pain (The chemistry of Aspirin (acetylsalicylic acid), 2020, para. 6). For this reason, Aspirin is commonly used to counter inflammatory response.
The most common application of Aspirin is pain mitigation. Being a nonsteroidal anti-inflammatory drug, Aspirin has no immunosuppressant effect because they attract immune cells, which decrease inflammation (Eid et al., 2021, p. 311). It is a usual remedy for headaches, toothaches, muscle aches, and other types of pain. Aspirin is also used for reduction of the impact of substances that cause swelling, inflammation or appearance of blood clots (Uzzaman, Chowdhury and Hossen, 2019, p. 1). Most importantly, Aspirin prevents heart attacks by affecting platelets. Unable to clump together, these cells cannot form blood clots. However, the downside to Aspirin is its effect on the stomach – not only does it cause irritation, but it can also lead to ulcers and bleeding.
After a person consumes a tablet of Aspirin, it follows into the digestive system. Starting with the esophagus, it travels to the stomach. In the stomach, the tablet dissolves into particles, which travel to the small intestine. There, most of the particles become absorbed into the blood. The blood stream carries the newly absorbed molecules throughout the entire body. First, the blood enriched by Aspirin travels to the liver, where some of the Aspirin particles are lost. Only after passing the liver does the bloodstream carry Aspirin to the site of pain. When the molecules arrive, they prevent the production of substances that cause pain and inflammation. The remaining Aspirin travels through the bloodstream and is sorted out again until no particles remain because of the excretion.
At each stage, the amount of Aspirin molecules travelling further is reduced. First, not all Aspirin particles are absorbed into the blood. When they pass through the liver, it filters the chemical input, reducing the subsequent effect on the body (Nokhodchi et al., 2022, p. 5). Then, ethanoic acid breaks off, leaving only salicylic acid. However, unlike ethanoic acid, the later cannot leave the body via urine. After undergoing conjugations, salicylic acid becomes glucuronide, which is water-soluble. As a result, a major part of the consumed Aspirin does not have any effect because of the extensive filtering. It is possible to bypass liver sorting by injecting aspirin intravenously.
In conclusion, Aspirin is a recognized and effective drug because it lifts pain, reduces inflammation and prevents blood clots. Acetylsalicylic acid is dissolved into smaller molecules, which are carried throughout the bloodstream, stopping pain and relieving inflammation. Furthermore, Aspirin’s ability to thin blood makes it an effective medication to prevent heart attack. On the downside, Aspirin can cause bleedings and have a negative effect on the stomach. Chemical reactions render a significant portion of Aspirin ineffective because of excretion. However, the use of intravenous injections can increase the efficiency of Aspirin consumption.
Reference List
Eid, R., et al. (2021) ‘Innate immune cell dysregulation drives inflammation and disease in aspirin-exacerbated respiratory disease’, Journal of Allergy and Clinical Immunology, 148(2), pp. 309-318.
Model list of essential medicines (2022) Web.
Nokhodchi, A., et al. (2022) ‘Solubility study of acetylsalicylic acid in ethanol+ water mixtures: measurement, mathematical modeling, and stability discussion’, AAPS PharmSciTech, 23(1), pp. 1-12.
Pahan, S. and Pahan, K. (2019) ‘Mode of action of aspirin in experimental autoimmune encephalomyelitis’, DNA and Cell Biology, 38(7), pp. 593-596.
The chemistry of Aspirin (acetylsalicylic acid) (2020) Web.
Uzzaman, M., Chowdhury, M.K. and Belal Hossen, M. (2019) ‘Thermochemical, molecular docking and ADMET studies of aspirin metabolites’, Frontiers in Drug, Chemistry and Clinical Research, 2, pp. 1-5.

