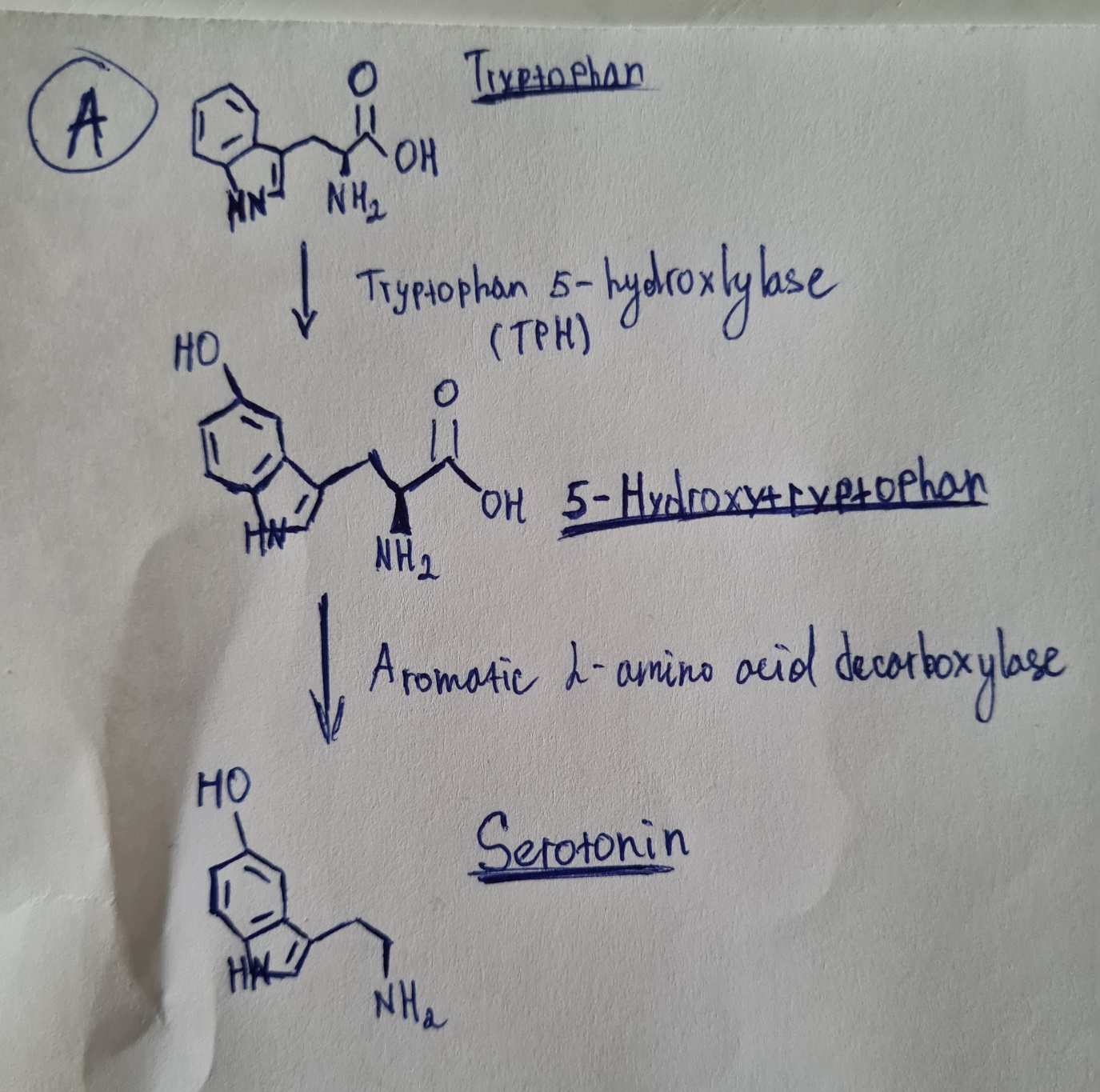
Various enzymes allow the conversion of tryptophan to serotonin. Similarly, some enzymes play a crucial role in the conversion of tyrosine to dopamine and subsequently to norepinephrine. In the diagram labeled by the letter “A,” the process of serotonin conversion is depicted. For serotonin produced peripherally and centrally, there is only one precursor, it is tryptophan (Colle et al., 2020).
Tryptophan is converted to 5-hydroxytryptophan, from which it transforms to serotonin, while serotonin is catabolized into 5-hydroxyindoleacetic acid (Colle et al., 2020). Thus, serotonin synthesis is bound to the availability of two enzymatic conversions and tryptophan (Colle et al., 2020). In the beginning, tryptophan hydroxylase 1 acts as a catalysator for the 5-hydroxytryptophan production from tryptophan. Then, 5-hydroxytryptophan via certain enzymes (aromatic amino acid decarboxylase) is converted to serotonin.
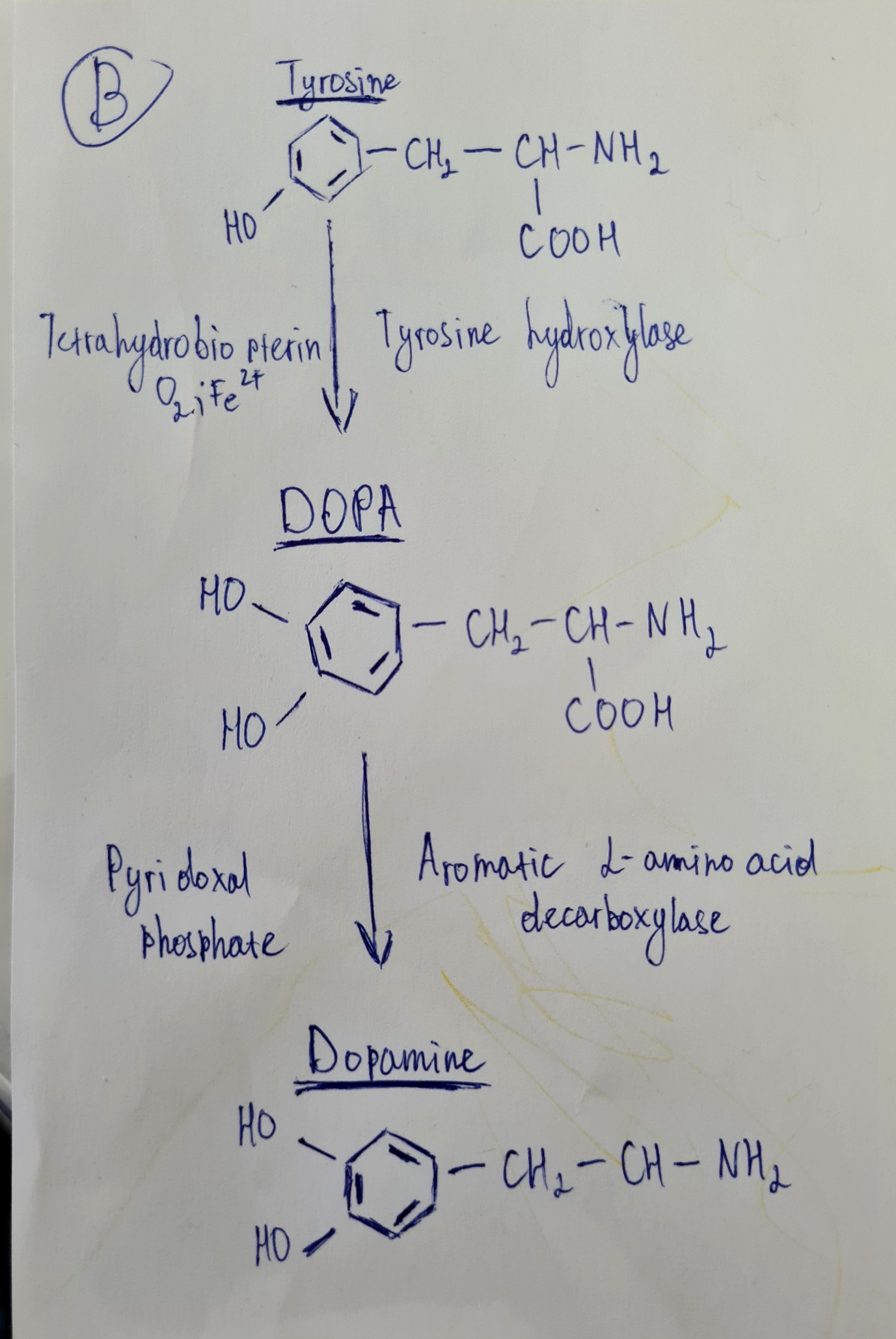
Dopamine is a neurotransmitter that a body utilizes to transmit messages between nerve cells. It is essential for the human perception of pleasure. Diagram B demonstrates the case of dopamine biosynthesis. The enzyme called tyrosine hydroxylase utilizes tetrahydrobiopterin in order to transform tyrosine into L-dihydroxyphenylalanine or L-DOPA (a.k.a. levodopa) (Zeng et al., 2020). L-DOPA is a large neutral amino acid (LNAA) and precursor for such catecholamines as dopamine, norepinephrine, and epinephrine (Zeng et al., 2020). Later follows the reaction during which aromatic L-amino acid decarboxylase continues the conversion of L-DOPA to dopamine.
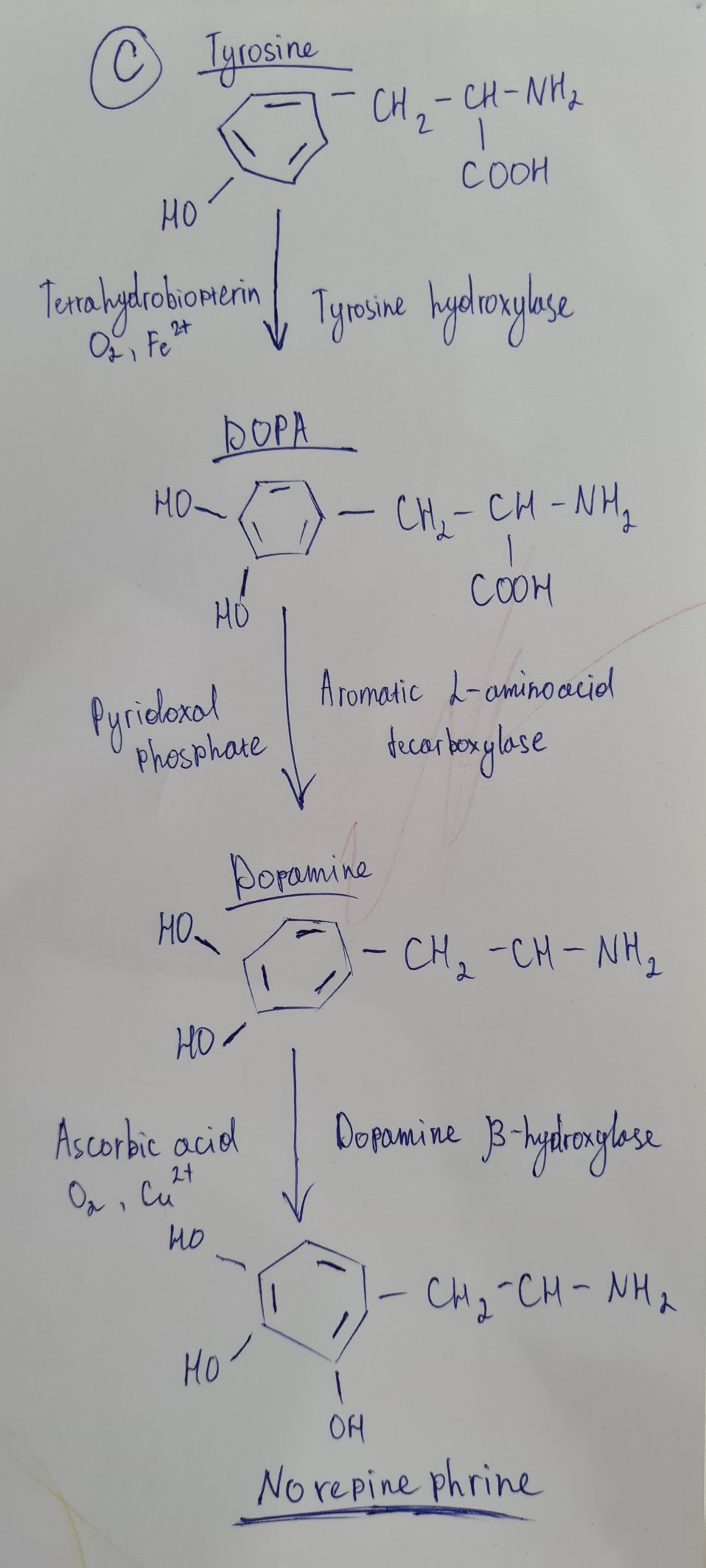
However, the process of conversion can continue to form norepinephrine. Norepinephrine has the function of a neurotransmitter and stress hormone. The latter function is observed during the time of stressful events perceived by the brain. In diagram C, it is evident that tyrosine undergoes the same process of conversion to DOPA and dopamine. Dopamine is then absorbed into vesicles, and conversion to the norepinephrine occurs. The conversion is done by dopamine β-hydroxylase enzymes which utilize ascorbic acid.
References
Colle, R., Masson, P., Verstuyft, C., Fève, B., Werner, E., Boursier‐Neyret, C.,… & Becquemont, L. (2020). Peripheral tryptophan, serotonin, kynurenine, and their metabolites in major depression: A case–control study. Psychiatry and clinical neurosciences, 74(2), 112-117
Zeng, B., Lai, Y., Liu, L., Cheng, J., Zhang, Y., & Yuan, J. (2020). Engineering Escherichia coli for high-yielding hydroxytyrosol synthesis from biobased L-tyrosine. Journal of Agricultural and Food Chemistry, 68(29), 7691-7696.

