Introduction
Memories and knowledge are essential to a personality, and acquiring new ones helps people change and develop. However, in some cases of injury and damage, amnesia occurs, and a person can forget existing memories and lose the ability to acquire new knowledge. In particular, anterograde amnesia (AA) suggests the inability to develop new declarative knowledge while maintaining the capabilities of procedural learning (Smith et al., 2013). Loss of memories before the incident, which disrupts memory, is characteristic of retrograde amnesia (RA) (Smith et al., 2013). RA often affects more recent memories preserving old ones, and can cover different periods depending on the severity of the injury (Smith et al., 2013). Both amnesia types can develop simultaneously in one patient with different severity of symptoms.
The nature of injuries that lead to AA or RA reveals the features of human memory and structures involved in remembering and recalling. The medial temporal lobe (MTL), also called the neocortex, and especially one of its structures, the hippocampus (HPC), has been at the center of the disputes and research on memory processes (Argyropoulos et al., 2022). The reason for the focus on these structures is the description of a case of patient HM, which became well-known in the scientific literature and subsequent evidence of these structures’ involvement (Argyropoulos et al., 2022). The current essay considers data from previous and recent studies on how damage to MTL and HPC affects memory processes.
Memory Structure
An essential aspect of understanding AA, RA, and memory specifics is memory structure and Ribot’s law or Ribot’s gradient. The law notes that with memory loss, recent memories are more affected than older ones, emphasizing that memory organization is time-dependent (Frankland & Bontempi, 2005). Moreover, studies of damage to MTL have shown that injuries to this structure can affect only part of memories, predominantly recent ones, which means that they gradually move to storage in other areas (Frankland & Bontempi, 2005). Consequently, older memories are solidified in other brain structures and unaffected by MTL processes.
Moreover, it is crucial to understand that different structures may be responsible for diverse information and experiences when considering memory processes. An indicative case of HM included the patient for whom a part of the hippocampus was removed to relieve epilepsy seizures (Duff et al., 2020). This situation and other similar cases showed that people lose the ability to remember events from their own lives, which is episodic memory, and acquire general knowledge – semantic memory; that is, they are unable to acquire declarative information (Frankland & Bontempi, 2005). However, the ability to acquire non-declarative knowledge, for example, through perceptual learning, has been preserved (Frankland & Bontempi, 2005). Thanks to such observations, it became clear that memory is not unitary but fragmented.
Memory Consolidation and Retrieval
HPC plays a crucial role in declarative memory as it is involved in consolidation. This process implies the transition of memories from a vulnerable state to a more stable one (Frankland & Bontempi, 2005). As shown in Figure 1, the human experience is encoded in HPC and cortical networks, leading to the formation of cortico-cortical connections (Frankland & Bontempi, 2005). Gradually, memories transit to the cortex and become independent of HPC (Fölsz et al., 2023).
The change in synaptic connections refers to fast consolidation, also called synaptic consolidation. Later, when memories become independent of HPC, neural circuits in the brain reorganize, which is a characteristic of systems consolidation, a slower process. AA and RA may have different severity in manifestations of symptoms. A study by Smith et al. (2013) states that when the MTL is harmed, AA is more common and must achieve a certain severity before RA begins to develop. Such a finding demonstrates that with injuries affecting MTL, the ability to receive new information is destroyed faster than memories that have already been consolidated.
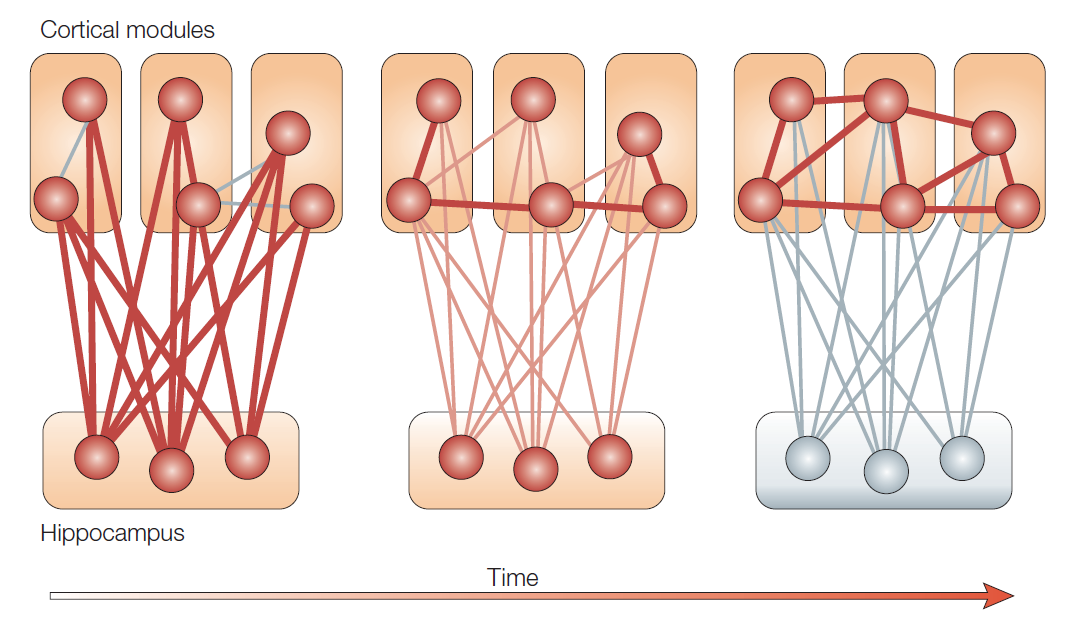
An essential process in memory consolidation for their subsequent retrieval is memory reactivation. Neural circuits that were active during the initial encoding are reactivated, strengthening cortical trace. Therefore, the HPC and cortex are active during this process since their circuits are remodeling. Frankland and Bontempi (2005) note that the ZIF268 gene regulates the reactivation process in the HPC, which is most commonly excreted during slow-wave sleep. Blocking this gene interferes with the stabilization of memories, again emphasizing the role of HPC in memory processes. In this way, reactivation in the HPC contributes to pattern completion, the ability to retrieve a complete memory from cues.
The additional role of MTL and HPC in memory processes is its retrieval. These structures are involved in object recognition; mainly, the perirhinal cortex, the MTL subregion, plays a crucial role in recognition (Argyropoulos, 2022). Smith et al. (2013) and Patterson et al. (2007) also argue that the temporal lobe is necessary for semantic memory, a subtype of declarative memory. Patterson et al. (2007) claim that the temporal lobes support the amodal hub responsible for the abilities associated with conceptual knowledge. Therefore, HPC and temporal lobes are critical for retrieval, and they engage in pattern completion, object recognition, and semantic memory retrieval. These processes involve interactions between various brain regions and complex neural computations.
Hippocampus and Amnesia
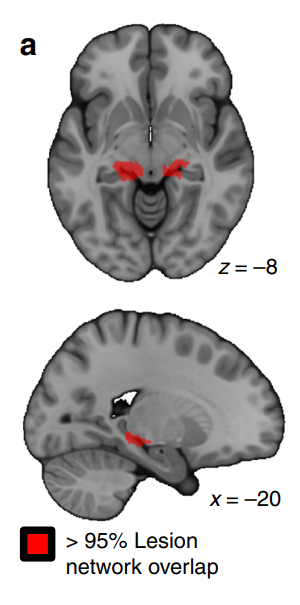
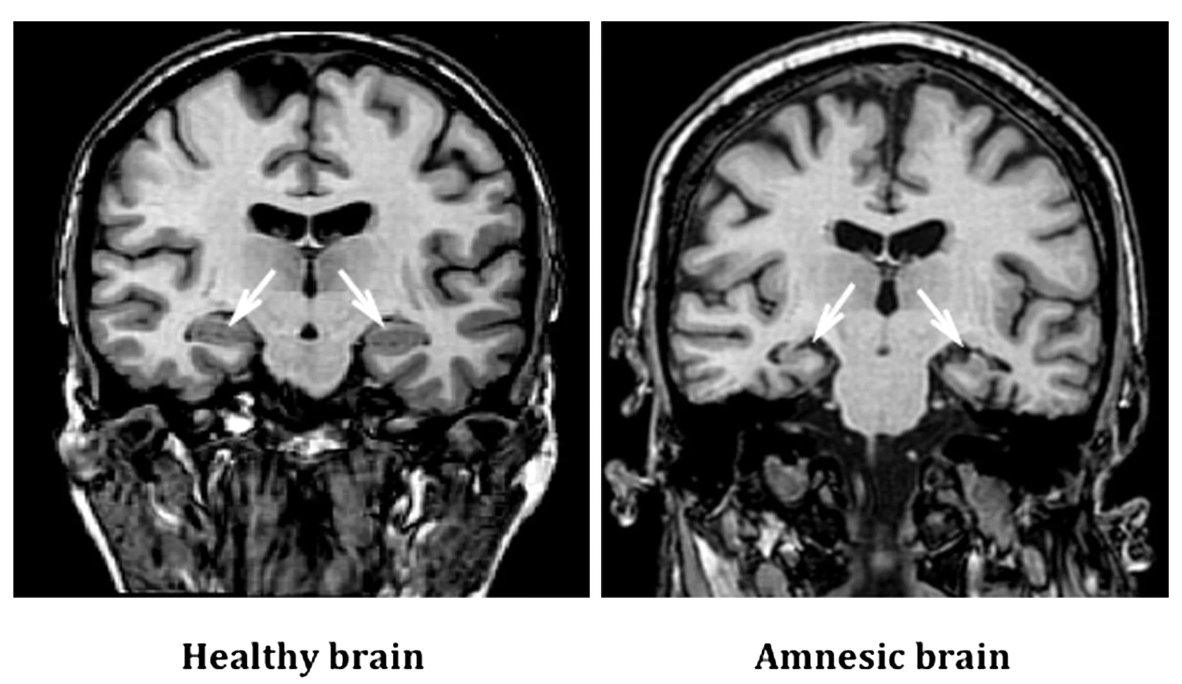
Given the role of MTL and HPC in memory, it is natural that the harm to these structures leads to amnesia. Several studies and cases of patients with amnesia confirm this association (Esteves et al., 2023; Ferguson et al., 2019). Ferguson et al. (2019) examined a sample of 53 patients with post-stroke amnesia. Figure 2 demonstrates that 95% of lesions leading to amnesia occurred in the hippocampus. Feinstein et al. (2010) study also confirms that hippocampal harm or atrophy leads to amnesia (Figure 3). Consequently, the critical memory circuit is located in the HPC, which confirms earlier assumptions about their relationship.
Conclusion
Thus, HPC and MTL are critical for creating and retrieving memories. The view prevails in research that the HPC is a temporary repository for acquired knowledge and memories. However, they are gradually consolidated, and their long-term storage depends on other systems. As a result, the damage caused to the HPC limits a person’s ability to gain new knowledge and memories, leading to AA. HPC is also critical in reactivation, which is necessary to strengthen memories for their subsequent retrieval. At the same time, part of the information is also stored in the temporal lobes. Therefore, injuries to the HPC and temporal lobes lead to RA, and the severity of harm can determine the gradient of the memories affected.
References
Argyropoulos, G. P. D., Dell’Acqua, C., Butler, E., Loane, C., Roca-Fernandez, A., Almozel, A., Drummond, N., Lage-Martinez, C., Cooper, E., Henson, R. N., & Butler, C. R. (2022). Functional specialization of the medial temporal lobes in human recognition memory: Dissociating effects of hippocampal versus parahippocampal damage. Cerebral Cortex, 32(8), 1637–1652. Web.
Duff, M. C., Covington, N. V., Hilverman, C., & Cohen, N. J. (2020). Semantic memory and the hippocampus: Revisiting, reaffirming, and extending the reach of their critical relationship. Frontiers in Human Neuroscience, 13, 1-17. Web.
Esteves, I. M., Chang, H., Neumann, A. R., & McNaughton, B. L. (2023). Consolidation of cellular memory representations in superficial neocortex. iScience, 26(2), 1-11. Web.
Feinstein, J. S., Duff, M. C., & Tranel, D. (2010). Sustained experience of emotion after loss of memory in patients with amnesia. Proceedings of the National Academy of Sciences, 107(17), 7674-7679. Web.
Ferguson, M. A., Lim, C., Cooke, D., Darby, R. R., Wu, O., Rost, N. S., Corbetta, M., Grafman, J., & Fox, M. D. (2019). A human memory circuit derived from brain lesions causing amnesia. Nature Communications, 10(1), 1-9. Web.
Fölsz, O., Trouche, S., & Croset, V. (2023). Adult-born neurons add flexibility to hippocampal memories. Frontiers in Neuroscience, 17, 1-9. Web.
Frankland, P. W., & Bontempi, B. (2005). The organization of recent and remote memories. Nature reviews. Neuroscience, 6(2), 119–130. Web.
Patterson, K., Nestor, P. J., & Rogers, T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nature reviews. Neuroscience, 8(12), 976–987. Web.
Smith, C. N., Frascino, J. C., Hopkins, R. O., & Squire, L. R. (2013). The nature of anterograde and retrograde memory impairment after damage to the medial temporal lobe. Neuropsychologia, 51(13), 2709–2714. Web.

