Introduction
Two drugs, lamotrigine and levetiracetam, are used to prevent epileptic strokes; they both are widely used and quite efficient. However, they also have side effects, including severe ones, which can appear in various conditions. While lamotrigine has a known mechanism of action and its side effects are predictable, levetiracetam can lead to severe drawbacks when administered in large doses, sometimes necessary to stop a stroke. In addition, lamotrigine is more cost-efficient and cheaper, and its effect is not worse than levetiracetam. An example of a patient who had severe side effects from levetiracetam will be provided, showing how they were overcome by changing the treatment to lamotrigine, whose effects are much easier to manage.
Epilepsy Overview and Its Treatment
Epilepsy is a neurological disorder that affects around 1 in 100 people worldwide. An injury or illness can cause it, but in most cases, it is inherited and runs in families. It leads to serious strokes, which damage one’s brain and can be treated by drugs that inhibit the brain areas, which are excessively active during epileptic strokes (Kessler & McGinnis, 2019; Winter et al., 2022). There are many types of epilepsy, and each person responds differently to treatment (Löscher et al., 2020; Moosa, 2019). For example, some people will only need one type of drug, while others may need two or three different types of medication at once to control their symptoms.
Two drugs, lamotrigine and levetiracetam, are widely used as first-line treatments for seizures, especially when other medications have failed. They both have known side effects; however, lamotrigine can be considered a safer and more cost-efficient option (Nevitt et al., 2018b). In this paper, various issues related to lamotrigine and levetiracetam prescription, such as cost-efficiency, side effects, and public health issues, will be explored, which will show that lamotrigine is a better option (Badarny, Badarny, and Mihilia, 2022; El-Haggar et al., 2018; Marson et al., 2021a; Marson et al., 2021b; Mikulić, Likić and Janković, 2022). An example of a patient with severe and unpredictable mental side effects from taking levetiracetam in high dosages will be provided.
Lamotrigine
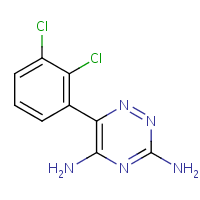
It is an anti-epileptic and anti-psychotic drug whose chemical formula can be seen in Figure 1. It is available in several forms, including tablets, chewable tablets, and orally disintegrating tablets. It is typically taken once or twice daily, and the dosage may be adjusted based on the patient’s needs and medication response (Naguy & Al-Enezi, 2019).
Like all medications, lamotrigine can have side effects, including dizziness, headache, blurred vision, and nausea (Parker, 2018). More severe effects include skin problems, known as Stevens-Johnson syndrome, which can still be managed by adjusting the dosage and learning the patient history (Edinoff et al., 2021). Therefore, lamotrigine has various side effects, including serious ones: they will be further described, and one will see that they can be managed.
Its mechanism of action, while still to be studied, is based on suppressing sodium channel activity. Specifically, it blocks voltage-sensitive sodium channels in the brain, preventing the excessive release of excitatory neurotransmitters and helping to control abnormal electrical activity in the brain that can cause seizures (Sills & Rogawski, 2020).
In addition, it reduces the release of glutamate, an excitatory neurotransmitter, and enhances the GABA release, an inhibitory neurotransmitter, in the brain (Naguy & Al-Enezi, 2019). It leads to reduced seizure activity, mood stabilization, and stroke ending, but it can also lead to brain activity suppression, which is the base of its primary side effects. In general, it is efficient in treating epilepsy and can be considered a first-line drug for this purpose, as its mechanism of action is quite understood, and its side effects are manageable.
Levetiracetam
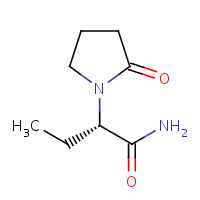
It is another anti-epileptic drug used to treat various types of seizures, including partial seizures, generalized seizures, and myoclonic seizures, and its structure is in Figure 2. It is also sometimes used as an off-label treatment for other conditions, such as neuropathic pain and anxiety. Levetiracetam is available in several forms, including tablets, extended-release tablets, and oral solutions. It is usually administered two times a day, with the dosage potentially modified depending on the patient’s requirements and how they respond to the medication (Sutherland et al., 2021).
It also has side effects, including drowsiness, dizziness, and nausea. In some cases, it can cause more serious side effects, such as behavioral changes, suicidal thoughts, or an allergic reaction (Badarny, Badarny, and Mihilia, 2022; Campbell et al., 2022; Dreischmeier et al., 2021). Therefore, it is similar to lamotrigine, but its side effects are more unpredictable (Josephson et al., 2019). As one will see, they can suddenly appear in case of dosage change and are connected with mental health problems.
The exact mechanism of action of levetiracetam is not fully understood, but it is believed to work by binding to a specific type of protein in the brain called synaptic vesicle protein 2A (SV2A). This protein is involved in releasing neurotransmitters, which are chemicals that help transmit signals between nerve cells (Litvinova et al., 2019; Löscher et al., 2020; Sills & Rogawski, 2020). By binding to SV2A, levetiracetam may help regulate neurotransmitters’ release and reduce the brain’s abnormal electrical activity that can cause seizures.
However, there are probably other aspects of its mechanism of action, and while they are unknown, they can be detrimental to a patient. There are cases when unknown side effects arose during the treatment, mostly connected with psychological issues. Thus, while levetiracetam is widely used and is quite manageable, it cannot be considered as safe as lamotrigine.
Ethical and Legislative Implication
All drugs should be legally approved before they can be administered, and in addition, most of them are available only by prescription. In the United Kingdom, lamotrigine is available by prescription only and is free if a patient has epilepsy (NHS, 2022a). It also approved treatment for bipolar disorder, migraine, and partial seizures with or without secondary generalization.
Levetiracetam is a relatively new drug, and many of its potential side effects are unknown now, similar to its clear mechanism of action; it is also available by prescription (NHS, 2022b). As with all prescription medications, both drugs are subject to strict regulations and prescribing guidelines to ensure their safe and effective use. They are recommended for use in case of pregnancy as being safer than other similar drugs (GOV.UK, 2021). However, as one will see, levetiracetam has problems of unpredictability, which may be detrimental to patients.
As was mentioned, much more is known about how lamotrigine can react during the treatment. While both drugs are available by prescription and are recommended in case of epilepsy, prescribers should know the mechanism of action and all possible drawbacks as well as possible. From an ethical point of view, the more is known about these, the lower the chance of adverse outcomes.
An example is a patient who came to the consultation with severe effects following levetiracetam usage. To stop an epileptic stroke, she needs to take various doses of the medicine, and in case of a dose change, adverse psychological side effects arise. She felt irritation, sudden mood changes, extreme dizziness, and even once lost consciousness.
While levetiracetam helped her with epilepsy, it eventually created more problems, and she was advised to change her medication to lamotrigine as a better-studied and manageable one. When her prescriber adjusted a dose for her, she started to take it, and no adverse effects were present. Therefore, ethically, it is better to prescribe well-studied medication, making lamotrigine a better option than levetiracetam.
Economic Issues
Drug cost-efficiency is measured as the drug value comparison with its cost: the more cost-efficient a drug is, the less money a patient will spend on it to recover. Cost efficiency can be evaluated by randomized controlled trials, which will show how the drug treats the problem and then compare it with the cost of its production and market prices (Marson et al., 2021b). Data about lamotrigine show that it is a good anti-psychotic drug, reducing not only epileptic strokes but also bipolar disorder psychoses (Naguy & Al-Enezi, 2019).
The cost of levetiracetam tends to be slightly higher than that of lamotrigine in most countries (Mikulić, Likić, and Janković, 2022). However, it does not show particularly greater efficiency, while its side effects are present and unpredictable (Aícua‐Rapún et al., 2019; Badarny, Badarny and Mihilia, 2022; El-Haggar et al., 2018). While there are various studies, most of them prove that lamotrigine has the best cost-efficiency and is superior to other drugs (Marson et al., 2021a; Marson et al., 2021b; Mikulić, Likić and Janković, 2022). Therefore, a patient who used to take levetiracetam not only suffered from adverse side effects but paid more for medication.
Psycho-Social Issues
Anti-epileptic drugs suppress the neural activity in the brain, and in certain conditions, they can have severe side effects. Common side effects of lamotrigine include dizziness, headache, insomnia, and nausea, especially in cases when such effects are typical for a patient (Liguori, Toledo, and Kothare, 2021). Serious side effects include rash or other allergic reactions (such as hives), liver damage (jaundice), blood disorders (e.g., low white blood cell count), and Stevens-Johnson syndrome (a severe skin reaction). They are present only in less than 1% of all cases and can be managed by dose adjustment (Edinoff et al., 2021). It must be used cautiously in people who have a history of Stevens-Johnson syndrome or other serious skin disorders.
Common side effects of levetiracetam include dizziness, drowsiness, and fatigue, similar to those of lamotrigine, which are common in 10% or more of all cases. Severe side effects may involve allergic reactions, liver damage, and blood disorders such as low white blood cell levels and kidney stone formation (Howard et al., 2018). Large doses greatly increase the chance of side effects, which can be unpredictable when such doses are necessary to stop severe epilepsy strokes (Howard et al., 2018; Mari et al., 2021).
Also, both drugs can decrease sperm quality in males, reducing fertility rates (Wu et al., 2018). In addition, levetiracetam is shown to harm bone formation, which is mostly not the case for lamotrigine (El-Haggar et al., 2018). While levetiracetam’s side effects are similar to lamotrigine in many ways, they may be unpredictable, and its prescriptions must be careful.
Therefore, both drugs show similar side effects, but lamotrigine ones are easier to manage. Both lamotrigine and levetiracetam are generally safe during pregnancy, but lamotrigine’s side effects are easier to predict and manage (Li & Meador, 2022). Doses should be lower during pregnancy to avoid problems with the fetus (Kaplan & Demir, 2021).
However, levetiracetam has more unusual and unpredictable side effects connected with mental health problems (Badarny, Badarny, and Mihilia, 2022). An example of a patient who suffered from severe physical issues after levetiracetam’s high doses shows how dangerous it can be. To summarize these issues, lamotrigine can be considered safer than levetiracetam, as its side effects are more predictable and no unwanted physical problems are present.
Public Health Issues
Last, public health issues are connected with prescribers developing patient-centered medication and management plans. Lamotrigine and levetiracetam are both prescribed in the same way by a healthcare professional. They can be taken by mouth, either on an empty stomach or with food (Celdran de Castro et al., 2023; Parker, 2018).
Both drugs are available as tablets and capsules, as well as liquids for oral suspension, and patients with epilepsy can obtain them for free as other necessary drugs (NICE, 2023a; NICE, 2023b). Typically, the medication is started at a low dose and gradually increased over time as needed to achieve the desired therapeutic effect. As one will see, it poses a serious risk in the case of levetiracetam, which is the reason why lamotrigine is shown as a better option.
Therefore, public health issues also include evaluating all prescription risks and working out how to avoid them. As mentioned, both drugs can cause side effects, and prescribers must be sure that there are no prerequisites for them in a patient. Lamotrigine may cause serious drawbacks such as a severe rash, movement problems, or a life-threatening allergic reaction called Stevens-Johnson syndrome (Edinoff et al., 2021; Jang et al., 2020; Parker, 2018; Rissardo & Fornari Caprara, 2021). To avoid them, a prescriber must carefully learn a patient’s medical history; most of them can be avoided by carefully adjusting the dosage. In addition, patients should be advised to follow the specific dosing instructions provided by a prescriber and not stop taking the medication abruptly without consulting.
In the case of levetiracetam, common side effects are dizziness, fatigue, and irritability, similar to those of lamotrigine, but it is known to cause severe psychological issues. They include behavioral changes, suicidal thoughts, or bone damage (Badarny, Badarny, and Mihilia, 2022; El-Haggar et al., 2018). In case of all drawbacks, patients should seek medical attention immediately if they experience any of these symptoms while taking levetiracetam and change the drug (Campbell et al., 2022; Celdran de Castro et al., 2023; Howard et al., 2018; Jarvie & Mahmoud, 2018; Josephson et al., 2019). As in the case of the consulted patient, she experienced severe behavioral changes when increasing her dosage. It is known that levetiracetam can cause such psychotic reactions, but they are unpredictable.
A Critical Reflection
Thus, the anti-epileptic drug prescription process faces many challenges: legislative, economic, psycho-social, and public health. Prescribers must count various factors, such as side effects, drug cost, laws connected with its usage, and all the latest studies in the area. As one can see, lamotrigine and levetiracetam have similar efficiency, but the former is cheaper and easier to manage.
Thus, lamotrigine can be considered a better option, as it is better researched, has fewer adverse side effects, and is more cost-efficient than levetiracetam. Its action mechanism is known better than levetiracetam; thus, the risk of unknown effects is lower. The patient who used levetiracetam suffered from many unpredictable side effects: when she was forced to increase her dosage, she suffered from psychotic problems, such as irritability and mental instability. Changing to lamotrigine enabled her to abolish these effects; as mentioned, there are similar cases, and one cannot know for sure about all possible drawbacks of levetiracetam. Therefore, it is better to use lamotrigine as the first-line anti-epileptic drug in cases when there are no clear signs that adverse side effects are possible.
Conclusion
Epilepsy can be treated by selectively suppressing the brain activity in areas where it is overly active during strokes, and lamotrigine is among the top choices for achieving this. Levetiracetam is another option: their mechanisms of action are different despite having similar clinical outcomes, but lamotrigine is easier to manage and more cost-efficient.
As the patient’s case showed, she suffered from unpredictable side effects of levetiracetam when it was necessary to take large doses: these effects included uncontrollable irritability and loss of consciousness. All these effects were abolished when she changed her medication to lamotrigine, without these adverse effects. While levetiracetam can improve the condition of epilepsy, and its effect is mostly similar to lamotrigine, the latter is safer and cheaper, and thus, it is preferred as a first-line epilepsy treatment option.
Reference List
Abou-Khalil, B.W. (2022). Update on antiseizure medications 2022. CONTINUUM: Lifelong Learning in Neurology, 28(2), pp.500–535. Web.
Aícua‐Rapún, I., André, P., Rossetti, A.O., Ryvlin, P., Hottinger, A.F., Decosterd, L.A., Buclin, T. and Novy, J. (2019). Therapeutic Drug Monitoring of Newer Antiepileptic Drugs: A Randomized Trial for Dosage Adjustment. Annals of Neurology, 87(1), pp.22–29. Web.
Badarny, S., Badarny, Y. and Mihilia, F. (2022). Republished: Unusual side effects of levetiracetam. Drug and Therapeutics Bulletin, p.dtb-2022-242496rep. Web.
Campbell, C., McCormack, M., Patel, S., Stapleton, C., Bobbili, D., Krause, R., Depondt, C., Sills, G.J., Koeleman, B.P., Striano, P., Zara, F., Sander, Josemir W., Lerche, H., Kunz, W.S., Stefansson, K., Stefansson, H., Doherty, C.P., Heinzen, E.L., Scheffer, I.E. and Goldstein, David B. (2022). A pharmacogenomic assessment of psychiatric adverse drug reactions to levetiracetam. Epilepsia, 63(6), pp.1563–1570. Web.
Celdran de Castro, A., Nascimento, F.A., Beltran-Corbellini, Á., Toledano, R., Garcia-Morales, I., Gil-Nagel, A. and Aledo-Serrano, Á. (2023). Levetiracetam, from broad-spectrum use to precision prescription: A narrative review and expert opinion. Seizure, 107, pp.121–131. Web.
Chamberlain, J.M., Kapur, J., Shinnar, S., Elm, J., Holsti, M., Babcock, L., Rogers, A., Barsan, W., Cloyd, J., Lowenstein, D., Bleck, T.P., Conwit, R., Meinzer, C., Cock, H., Fountain, N.B., Underwood, E., Connor, J.T., Silbergleit, R., Gray, E. and Gunter, S. (2020). Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. The Lancet, 395(10231), pp.1217–1224. Web.
Dreischmeier, E., Zuloaga, A., Kotloski, R.J., Karasov, A.O. and Gidal, B.E. (2021). Levetiracetam-associated irritability and potential role of vitamin B6 use in veterans with epilepsy. Epilepsy & Behavior Reports, 16. Web.
EBI. (2017). levetiracetam (CHEBI:6437). Web.
EBI. (2021). lamotrigine (CHEBI:6367). Web.
Edinoff, A.N., Nguyen, L.H., Fitz-Gerald, M.J., Crane, E., Lewis, K., Pierre, S.S., Kaye, A.D., Kaye, A.M., Kaye, J.S., Kaye, R.J., Gennuso, S.A., Varrassi, G., Viswanath, O. and Urits, I. (2021). Lamotrigine and Stevens-Johnson Syndrome Prevention. Psychopharmacology bulletin, 51(2), pp.96–114. Web.
El-Haggar, S.M., Mostafa, T.M., Allah, H.M.S. and Akef, G.H. (2018). Levetiracetam and lamotrigine effects as mono- and polytherapy on bone mineral density in epileptic patients. Arquivos de Neuro-Psiquiatria, 76(7), pp.452–458. Web.
Fiani, B., Andraos, C., Mabry, I. and Siddiqi, J. (2021). A Comparison of Seizure Prophylaxis: Phenytoin Versus Levetiracetam. Cureus, 13(5). Web.
GOV.UK. (2021). Antiepileptic drugs in pregnancy: updated advice following comprehensive safety review. Web.
Hakami, T. (2021). Neuropharmacology of antiseizure drugs. Neuropsychopharmacology Reports, 41(3), pp.336–351. Web.
Haller, J.T., Bonnin, S. and Radosevich, J. (2021). Rapid administration of undiluted intravenous levetiracetam. Epilepsia, 62(8), pp.1865–1870. Web.
Han, Y., Yang, J., Zhong, R., Guo, X., Cai, M. and Lin, W. (2022). Side effects of long-term oral anti-seizure drugs on thyroid hormones in patients with epilepsy: a systematic review and network meta-analysis. Neurological Sciences, 43(9). Web.
Howard, P., Remi, J., Remi, C., Charlesworth, S., Whalley, H., Bhatia, R., Hitchens, M., Mihalyo, M. and Wilcock, A. (2018). Levetiracetam. Journal of Pain and Symptom Management, 56(4), pp.645–649. Web.
Jang, Y., Moon, J., Son, H., Lee, W.-J., Sunwoo, J.-S., Lee, S.-T., Park, K.-I., Jeon, D., Chu, K. and Lee, S.K. (2020). A new rapid titration protocol for lamotrigine with reduced risk of skin rash. The Journal of Immunology, 204(1_Supplement), pp.64.30–64.30. Web.
Jarvie, D. and Mahmoud, S.H. (2018). Therapeutic Drug Monitoring of Levetiracetam in Select Populations. Journal of Pharmacy & Pharmaceutical Sciences, 21(1s), pp.149s176s. Web.
Josephson, C.B., Engbers, J.D.T., Jette, N., Patten, S.B., Singh, S., Sajobi, T.T., Marshall, D., Agha-Khani, Y., Federico, P., Mackie, A., Macrodimitris, S., McLane, B., Pillay, N., Sharma, R. and Wiebe, S. (2019). Prediction Tools for Psychiatric Adverse Effects after Levetiracetam Prescription. JAMA Neurology, 76(4), p.440. Web.
Kanner, A.M. and Bicchi, M.M. (2022). Antiseizure medications for adults with epilepsy. JAMA, 327(13), p.1269. Web.
Kaplan, Y.C. and Demir, O. (2021). Use of phenytoin, phenobarbital carbamazepine, levetiracetam lamotrigine and valproate in pregnancy and breastfeeding: Risk of major malformations, dose-dependency, monotherapy vs polytherapy, pharmacokinetics and clinical implications. Current Neuropharmacology, 19. Web.
Kapur, J., Elm, J., Chamberlain, J.M., Barsan, W., Cloyd, J., Lowenstein, D., Shinnar, S., Conwit, R., Meinzer, C., Cock, H., Fountain, N., Connor, J.T. and Silbergleit, R. (2019). Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. New England Journal of Medicine, 381(22), pp.2103–2113. Web.
Kessler, S.K. and McGinnis, E. (2019). A practical guide to treatment of childhood absence epilepsy. Pediatric Drugs, 21(1), pp.15–24. Web.
Li, R., Zhou, Q., Ou, S., Wang, Y., Li, Y., Xia, L. and Pan, S. (2020). Comparison of long-term efficacy, tolerability, and safety of oxcarbazepine, lamotrigine, and levetiracetam in patients with newly diagnosed focal epilepsy: An observational study in the real world. Epilepsy Research, 166, p.106408. Web.
Li, Y. and Meador, K.J. (2022). Epilepsy and pregnancy. CONTINUUM: Lifelong Learning in Neurology, 28(1), pp.34–54. Web.
Liguori, C., Toledo, M. and Kothare, S. (2021). Effects of anti-seizure medications on sleep architecture and daytime sleepiness in patients with epilepsy: A literature review. Sleep Medicine Reviews, 60, p.101559. Web.
Litvinova, S.A., Voronina, T.A., Nerobkova, L.N., Kutepova, I.S., Avakyan, G.G. and Avakyan, G.N. (2019). Levetiracetam effect and electrophysiological mechanism of action in rats with cobalt-induced chronic epilepsy. European Journal of Pharmacology, 854, pp.380–386. Web.
Liu, B.-K., Jiang, L., Li, X.-J., Hong, S.-Q., Chen, W. and Hu, Y. (2019). Efficacy and safety of levetiracetam in the off-label treatment of neonatal seizures. International Journal of Neuroscience, 130(4), pp.336–342. Web.
Löscher, W., Potschka, H., Sisodiya, S.M. and Vezzani, A. (2020). Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacological Reviews, 72(3), pp.606–638. Web.
Mari, L., Placidi, F., Romigi, A., Tombini, M., Del Bianco, C., Ulivi, M., Liguori, C., Manfredi, N., Castelli, A., Mercuri, N.B. and Izzi, F. (2021). Levetiracetam, lamotrigine and carbamazepine: which monotherapy during pregnancy? Neurological Sciences, 43(3). Web.
Marson, A., Burnside, G., Appleton, R., Smith, D., Leach, J.P., Sills, G., Tudur-Smith, C., Plumpton, C., Hughes, D.A., Williamson, P., Baker, G.A., Balabanova, S., Taylor, C., Brown, R., Hindley, D., Howell, S., Maguire, M., Mohanraj, R., Smith, P.E. and Lanyon, K. (2021a). The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. The Lancet, 397(10282), pp.1363–1374. Web.
Marson, A., Burnside, G., Appleton, R., Smith, D., Leach, J.P., Sills, G., Tudur-Smith, C., Plumpton, C., Hughes, D.A., Williamson, P., Baker, G.A., Balabanova, S., Taylor, C., Brown, R., Hindley, D., Howell, S., Maguire, M., Mohanraj, R., Smith, P.E. and Lanyon, K. (2021b). The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. The Lancet, 397(10282), pp.1375–1386. Web.
Mikulić, I., Likić, R. and Janković, S.M. (2022). Cost-Effectiveness of Zonisamide versus Levetiracetam in Newly Diagnosed Focal Onset Epilepsy in Serbia. Value in Health Regional Issues, 27, pp.49–57. Web.
Moosa, A.N.V. (2019). Antiepileptic drug treatment of epilepsy in children. CONTINUUM: Lifelong Learning in Neurology, 25(2), pp.381–407. Web.
Naguy, A. and Al-Enezi, N. (2019). Lamotrigine uses in psychiatric practice. American Journal of Therapeutics, 26(1), pp.e96–e102. Web.
Nevitt, S.J., Sudell, M., Weston, J., Tudur Smith, C. and Marson, A.G. (2018a). Antiepileptic drug monotherapy for epilepsy: A network meta-analysis of individual participant data. Cochrane Database of Systematic Reviews. Web.
Nevitt, S.J., Tudur Smith, C., Weston, J. and Marson, A.G. (2018b). Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review. Cochrane Database of Systematic Reviews, 2018(6). Web.
NHS. (2022a). About lamotrigine. Web.
NHS. (2022b). Common questions about levetiracetam. Web.
NICE. (2023a). BNF: Lamotrigine medicinal forms. Web.
NICE. (2023b). BNF: Levetiracetam medicinal forms. Web.
Parker, G. (2018). Risks associated with lamotrigine prescription: A review and personal observations. Australasian Psychiatry, 26(6), pp.640–642. Web.
Peter-Derex, L., Philippeau, F., Garnier, P., André-Obadia, N., Boulogne, S., Catenoix, H., Convers, P., Mazzola, L., Gouttard, M., Esteban, M., Fontaine, J., Mechtouff, L., Ong, E., Cho, T.-H., Nighoghossian, N., Perreton, N., Termoz, A., Haesebaert, J., Schott, A.-M. and Rabilloud, M. (2022). Safety and efficacy of prophylactic levetiracetam for prevention of epileptic seizures in the acute phase of intracerebral haemorrhage (PEACH): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Neurology, 21(9), pp.781–791. Web.
Ricci, L., Croce, P., Pulitano, P., Boscarino, M., Zappasodi, F., Narducci, F., Lanzone, J., Sancetta, B., Mecarelli, O., Di Lazzaro, V., Tombini, M. and Assenza, G. (2022). Levetiracetam Modulates EEG Microstates in Temporal Lobe Epilepsy. Brain Topography, 35(5-6), pp.680–691. Web.
Rissardo, J. and Fornari Caprara, A. (2021). Lamotrigine-Associated movement disorder: A literature review. Neurology India, 69(6), p.1524. Web.
Sills, G.J. and Rogawski, M.A. (2020). Mechanisms of action of currently used antiseizure drugs. Neuropharmacology, 168, p.107966. Web.
Sutherland, A., Meldon, C., Harrison, T. and Miller, M. (2021). Subcutaneous Levetiracetam for the Management of Seizures at the End of Life: An Audit and Updated Literature Review. Journal of Palliative Medicine, 24(7), pp.976–981. Web.
Winter, Y., Uphaus, T., Sandner, K., Klimpe, S., Stuckrad-Barre, S. von and Groppa, S. (2022). Efficacy and safety of antiseizure medication in post-stroke epilepsy. Seizure, 100, pp.109–114. Web.
Wu, D., Chen, L., Ji, F., Si, Y. and Sun, H. (2018). The effects of oxcarbazepine, levetiracetam, and lamotrigine on semen quality, sexual function, and sex hormones in male adults with epilepsy. Epilepsia, 59(7), pp.1344–1350. Web.

