Introduction
It is important to note that brain tissue is among the most complex and intricate structures of the human body, which has a highly limited capacity for healing and regeneration. The topic of the proposed research is to assess how neural stem cells replace and differentiate into neurons and other cells of the brain to use them for therapeutic purposes. Because of their capacity for differentiation and rejuvenation, brain stem cells must be employed therapeutically to slow or stop the aging process.
Literature Review
The topic of neural stem cells is a complex and intricate subject, but its applicability and utility can be massive and widespread, with the capability to address the most critical health issues revolving around brain-related diseases and aging. The current literature is extensive and provides sufficient evidence favoring the proposed thesis, where a wide range of methods can be used to use stem cells or SC as a therapeutic measure. The general research suggests that there is a complex system of regulatory steps that involve GABA signaling. There is an array of signaling molecules involved in the ventricular-subventricular zone (V-SVZ) neurogenesis, such as Sonic hedgehog, fibroblast growth factor 2, glutamate, and others. The subgranular zone (SGZ) is another important germinal region of the adult human brain, and the mechanisms of regulation are comparatively different between V-SVZ and SGZ.
To assess the details of the use of neural stem cells, it is important to understand the general state of knowledge in the field of interest. Summative research on the promising uses of neural stem cells or NSCs suggests that the primary uses lie in “investigating embryonic neurogenesis, modeling of the pathogenesis of diseases of the central nervous system, and designing drug-screening systems” (Galiakberova & Dashinimaev, 2020, p. 1). In other words, the study explores how neural stem cells differentiate into different forms of mature neural cells, including neurons themselves. Pluripotent stem cells or PSCs are the best candidates for in vitro generation and cultivation of neural stem cells. A rigorous condition setup is essential since the differentiation process involves a wide range of phenotypes.
When it comes to neural stem cells, neurogenesis is a central process that is heavily reliant on the functionality of the hippocampal region. A study states that “as production of new neurons decreases after birth, purposefully activating stem cells to create additional new neurons may augment brain function or slow a disease’s progression” (Lazutkin et al., 2019, p. 1). The argument is made that “the basic blueprint of how stem cells are maintained, divide, differentiate, and are eliminated is still contentious, with different models potentially leading to vastly different outcomes regarding neuronal production and stem cell pool preservation” (Lazutkin et al., 2019, p. 1). Therefore, the research analyzes the process of hippocampal neurogenesis, where the emphasis is put on the neural stem cell lifecycle. The proposed models of neural stem cell maintenance, division, and differentiation can occur in both symmetric and asymmetric ways. The maintenance of brain stem cells, including quiescent ones, is largely dependent on astrocytes.
In addition to hippocampal involvement in neural stem cell generation, two key zones within this brain unit also need to be analyzed. These mammalian brain regions include the subventricular zone (SVZ) and dentate gyrus (DG) (Navarro Negredo et al., 2020). It is reported that the “mechanisms that regulate neural stem cells (NSCs) during aging, focusing on the effect of metabolism, genetic regulation, and the surrounding niche. We also explore emerging rejuvenating strategies for old NSCs” (Navarro Negredo et al., 2020, p. 202). In other words, the study focuses on two key neural stem cell pools in the mammalian brain, which include the subventricular zone and dentate gyrus. While DG is found in the hippocampus, SVZ is found in the lateral ventricles. The assessment reveals that neural stem cells are critical for brain rejuvenation, and health-promoting interventions slow brain aging.
One of the key pieces of literature relevant to the topic of interest is centered around neural stem cell differentiation and specialization. It is found that “V-SVZ NSCs are regulated by local signals from their immediate neighbors, as well as by neurotransmitters and factors that are secreted by distant neurons, the choroid plexus, and vasculature” (Obernier & Alvarez-Buylla, 2019, p. 1). In other words, distantly located cells secrete a wide range of signaling molecules to regulate the neural stem cell generation, differentiation, and regeneration processes. Thus, the study focuses on the process of cell specialization and differentiation in the rodent brain. Regional specificity, heterogeneity, and molecular control are the essential components. Neurotransmitters are said to be used by nearby cells to communicate the pattern of neural stem cell self-renewal.
Although the aging process halts the active phase of brain development and cell renewal, evidence suggests that adult neural stem cells or aNSCs are still impactful in sustaining the brain tissue. However, these stem cells cannot be grouped into one group because there is a “heterogeneity of aNSCs, including short-term and long-term self-renewing aNSCs, regional and temporal differences in aNSC function, and single-cell transcriptomics” (Petrik et al., 2022, p. 1). In other words, the study specifically focuses on neural stem cells in adults because they produce new neurons throughout one’s life. The authors address the recent findings regarding progenitor cells being able to undergo self-renewal. Through cell replacement, the factor of heterogeneity is essential to long-term brain health.
The investigated therapeutic process cannot be fully understood and comprehensively formulated without addressing the utility of transplantation procedures. It is stated that “cell therapy based on NSC transplantation is a promising tool for the treatment of nervous system diseases” (Tang et al., 2017, p. 1). Thus, it is critical to provide a summative overview of transplantation procedures regarding neural stem cells. There are several problems with the field’s present advancement. It is indicated that novel derivation methods are developed, such as trans-differentiation from somatic cells.
It should be noted that adult neural stem cells’ activity is heavily impacted by the quiescence or so-called state of reversible cell cycle arrest. It is reported that “the balance between stem cell quiescence and activity determines not only the rate of neurogenesis but also the long-term maintenance of the stem cell pool and the neurogenic capacity of the aging brain” (Urbán et al., 2019, p. 834). In other words, the aging of the brain is in direct relationship with two different states of neural stem cells, which can be triggered to switch through a range of physiological stimuli.
One should be aware that brain aging and activity levels of neural stem cells are impacted by genetic factors. A study indicates that “NSCs are regulated by both intrinsic genetic and epigenetic programs and extrinsic stimuli transduced through the stem cell niche, dysregulation of NSCs due to either genetic causes or environmental impacts may lead to disease” (Zhao & Moore, 2018, p. 1). Therefore, the regulatory understanding of neural stem cells cannot exclude the pressures made by genetic elements, which means that not only internal brain physiology is critical, but also gene expression levels.
Brain Aging
Based on the literature analyzed above, it is evident that both the subventricular zone and dentate gyrus, as well as the hippocampus in general, play a critical role in regulating the brain aging processes. It is reported that “NSCs have the potential to rejuvenate old brains and ameliorate age-related neurodegenerative diseases” (Navarro Negredo et al., 2020, p. 215). An overwhelming amount of evidence suggests that neural stem cells can be highly effective and play a significant role in slowing not only aging but also rejuvenation. The traditional means of health improvements through exercise, dietary management, and blood factors tend to impact the aging slow down. However, genetic factors also determine the impactful of neural stem cells on the aging process. For example, experiments on mice show that “differences in spatial memory function between individual old rats are positively correlated with newborn neuron numbers in the hippocampus” (Navarro Negredo et al., 2020, p. 216). Therefore, neural stem cells’ initial count is determined by genes and development, but these different factors can be improved through stem cell therapies.
Neural Stem Cell Differentiation
In the case of using neural stem cells as a therapeutic measure to address many issues regarding the human brain, it is important to understand how these cells differentiate into mature neural cells and neurons. Most neural stem cells are located in the subventricular zone and dentate gyrus of the hippocampus. The stated regions are primarily regulated through local signals from the proximal locations in the brain, which reciprocally induce or inhibit the differentiation processes. Figure 1 below illustrates how neurogenesis with differentiation occurs, where the chorus plexus (CP) secretes stimuli factors in the lateral ventricle (LV) to facilitate neuron development in the ventricular zone (VZ) and SVZ. BV denotes blood vessels, and N refers to mature neurons. However, the most interesting cells include ependymal cells or E/E2, B1, B2, C, and astrocytes or A (Obernier & Alvarez-Buylla, 2019). The complex of cells is heavily involved in the regulation of neural stem cell differentiation.
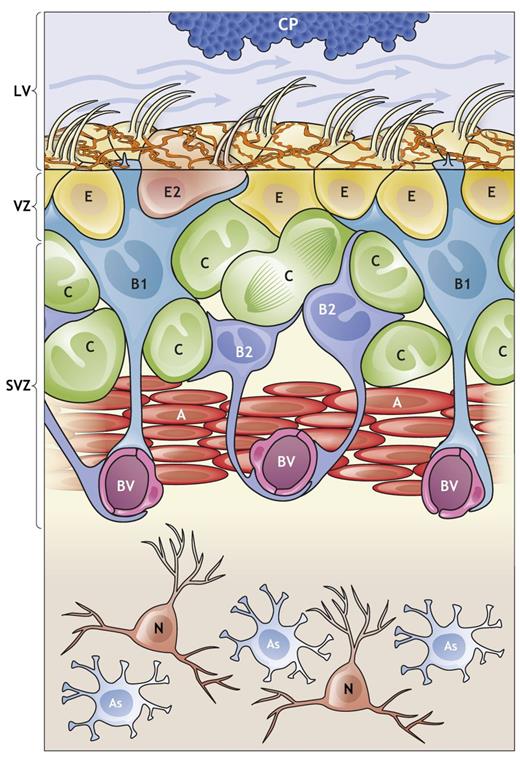
Differentiation Regulation
Since it was established that neural stem cell regulation is impacted by the intricate relationship of various cell types within V-SVZ, the dynamics of influence between them need to be investigated. It has been found that “the diazepam-binding inhibitor protein (DBI) from B1 cells and C cells increases V-SVZ proliferation and A cell production” (Obernier & Alvarez-Buylla, 2019, p. 4). However, astrocytes or “A cells release gamma-aminobutyric acid (GABA), which activates GABAA-receptors on B1 cells, thereby reducing proliferation” (Obernier & Alvarez-Buylla, 2019, p. 4). Therefore, CP releases BMP5 to upregulate differentiation on B, where A cells release GABA to induce C cells to facilitate stem cell maturation. Although the provided framework of analysis needs to consider a wider pool of factors impacting these changes, GABA and BMP5 need to be highlighted as prime inducers in V-SVZ.
Therapeutic Approaches
The research described and analyzed above strongly points toward the applicability of therapeutic measures to use neural stem cells for a wide range of purposes. It is stated that “single-cell RNA-sequencing and lineage tracing approaches could also help to understand regional differences among B1 cells” (Obernier & Alvarez-Buylla, 2019, p. 11). In addition, the therapy needs to use methods such as “single-cell RNA-seq, single-cell, ATAC-seq, or single-cell proteomics” to “capture a snapshot of the genomic, epigenomic, proteomic, and metabolomic landscapes of individual cells in a single assay” (Navarro Negredo et al., 2020, p. 216). CRISPR technology can also be used in the proposed therapeutic process to conduct a detailed genetic screening to understand the inborn predisposition of a person towards aging and brain rejuvenation. Aside from transplantation of new neural stem cells, sequencing, CRISPR, and the use of GABA and BMP5 in V-SVZ, different lineages of aNSCs can be targeted to further induce rejuvenation with a higher degree of precision and less reliance on the external introduction of cells (Petrik et al., 2022, p. 1). Therefore, rejuvenation and aging halting can be achieved through a combination of these methodological approaches.
Discussion
The differentiation observed in vitro with the expression of markers of mature cells does not yet prove the possibility of the replacement of specialized cells in vivo. For example, the development of a neuron-like phenotype in SC culture is explained by changes in the cytoskeleton under the action of biologically active substances added to the culture medium. There are no standard guidelines for obtaining and clinical use of SCs. Genetic instability, immuno-, and tumorigenic potential limit their clinical use. Genetic and epigenetic disorders that occur during cell culture can give them carcinogenic properties. From the general pathology, it is known that local cell proliferation leads to the growth of the nodule and not to the migration of individual cells into damaged tissues, where they are expected to be needed.
Conclusion
In conclusion, because of their capacity for differentiation and rejuvenation, brain stem cells must be employed therapeutically to reduce or stop the aging process. The therapeutics need to utilize new neural stem cells, sequencing, CRISPR, the use of GABA and BMP5 in V-SVZ, and different lineages of aNSCs. Therefore, there are several possibilities despite the limitations contained in each measure. It is critical to combine these frameworks of brain aging to slow down and rejuvenate on an individualized basis with proper screening procedures.
References
Galiakberova, A. A., & Dashinimaev, E. B. (2020). Neural stem cells and methods for their generation from induced pluripotent stem cells in vitro. Frontiers in Cell and Developmental Biology, 8, 1-20. Web.
Lazutkin, A., Podgorny, O., & Enikolopov, G. (2019). Modes of division and differentiation of neural stem cells. Behavioural Brain Research, 374, 1-9. Web.
Navarro Negredo, P., Yeo, R. W., & Brunet, A. (2020). Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell, 27(2), 202-223. Web.
Obernier, K., & Alvarez-Buylla, A. (2019). Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development, 146(4), 1-15. Web.
Petrik, D., Jorgensen, S., Eftychidis, V., & Siebzehnrubl, F. A. (2022). Singular adult neural stem cells do not exist. Cells, 11(722), 1-22. Web.
Tang, Y., Yu, P., & Cheng, L. (2017). Current progress in the derivation and therapeutic application of neural stem cells. Cell Death and Disease, 8(10), 1-12. Web.
Urbán, N., Blomfield, I. M., & Guillemot, F. (2019). Quiescence of adult mammalian neural stem cells: A highly regulated rest. Neuron, 104(5), 834–848. Web.
Zhao, X., & Moore, D. L. (2018). Neural stem cells: Developmental mechanisms and disease modeling. Cell and Tissue Research, 371(1), 1–6. Web.
